Below is our recent interview with Kamal Kumawat, Digital Marketing Manager at Johari Digital:
Q: Could you provide our readers with a brief introduction to your company?
A: Johari Digital is a global medical device Design, Development, and Manufacturing services provider with 40+ years of experience across FDA Class I & II products. Our manufacturing facility complies with ISO 13485:2016 and FDA (21 CFR 820), MDSAP, BIS standards, and processes. Johari caters to global clients from a 65,000 sq. ft., state-of-the-art medical device manufacturing facility in India along with an R&D Centre in Serbia & Hyderabad (India) and a Business Development team in India & USA. To date, Johari has 18 US FDA 510 (k) Certifications, successful commercialization of 145+ medical devices, and 100+ active clients worldwide (80% of their client portfolio is from the US).
Q: Any highlights on your recent announcement?
A: Recently, Myolift QT manufactured by Johari Digital Healthcare Ltd received US FDA 510 (k) clearance for OTC. The device is indicated for Facial and Neck Electrostimulation along with over-the-counter cosmetic uses. The clearance also includes Mobile APP, which gives the device a huge competitive edge. Additionally, four Electrodes and Conductive gloves are included in the application. With this achievement, Myolift QT owned by 7e Wellness, USA can successfully reach multiple global markets.

Recommended: An Interview With Samuel Perry, The Founder At Jam House
Q: Can you give us more insights into your offering?
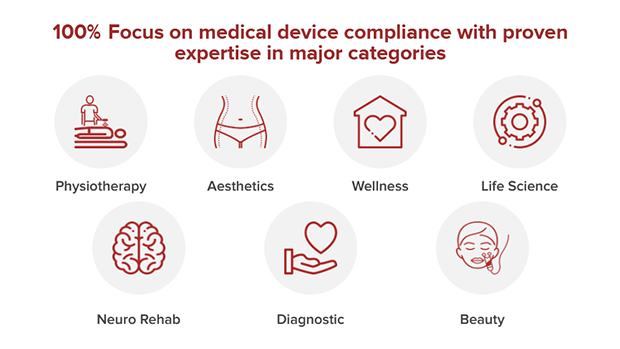
A: The company assists clients with End-to-End Medical Device Manufacturing & Development Services. Additionally, Johari also helps clients with regulatory compliance support to quickly take their products to market. Basically, we’re a Full Turnkey Medical Device Manufacturer providing comprehensive Medical Device Contract Manufacturing and Design/Regulatory (CE, US FDA & more) support services under one roof. Our manufacturing and development services span across categories of hospital equipment & critical care devices, vital monitoring devices, imaging systems, medical aesthetic devices, in-vitro diagnostics, wearable medical devices, point-of-care diagnostics, ultrasound, electrotherapy, Neuro-stimulation, artificial intelligence, and Bluetooth- and cloud-based medical devices.
Q: What can we expect from your company in next 6 months? What are your plans?
A: In the next 6 Months, we’re expanding our manufacturing facility and we’re consistently incorporating automation at different levels including the production floor. Under the banner of “Make in India” we aim to onboard more OEMs/Global Medical Device Companies to align with us as their Manufacturing & development partner. Currently, we’re focused on Multi-fold scaling and bringing many more innovative medical device breakthroughs to the market.
Recommended: Chatham Confirms Significant Progress In Its Monocalcium Phosphate Project
Q: What is the best thing about your company that people might not know about?
A: The best thing is that we work with our clients throughout the development cycle. Unlike other Contract Manufacturers, we don’t limit ourselves to said services, in many instances we adapt our systems and take extra steps to assist clients in taking their products successfully to global markets. We offer sustainable engineering services and work collaboratively with clients to ensure cost-optimized, regulatory-compliant, and successful Go-To-Market of Medical Devices.