Below is our recent interview with Ram Bhatt, the Founder and CEO at ICB International, Inc:
Q: Could you provide our readers with a brief introduction to your company?
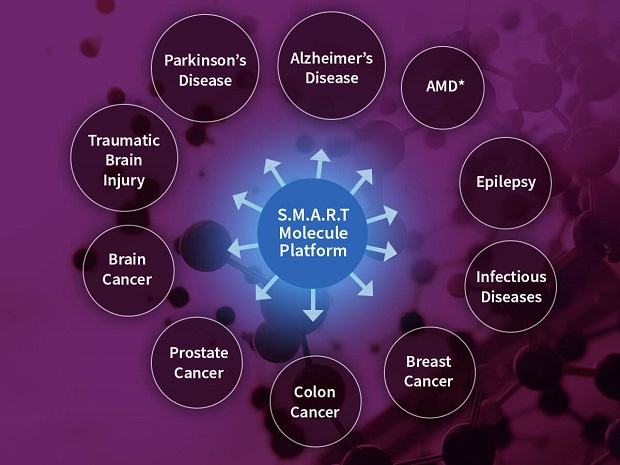
A: ICBII: La Jolla, California based ICB International, Inc., (“ICBII”), was founded in 2008 for the sole purpose of solving the single biggest problem that has stymied development of treatments for neurological diseases including, but not limited to, Alzheimer’s (AD), Parkinson’s (PD), Multiple System Atrophy (MSA), and Glioblastoma (GBM). Quite simply, you cannot treat a disease if medicines cannot reach the target site of the disease. For neurological diseases, this problem occurs because of a blood-brain barrier (BBB) that exists in all humans, which blocks nearly 98% of all pharmaceutical drugs from reaching the central nervous system. ICBII has developed a proven technology called SMART Molecules that can cross the blood-brain-barrier very efficiently and target specific disease-causing targets in the brain. The Company was founded by biotech veteran Ram Bhatt, PhD, who has more than 30 years’ experience in developing innovative technologies for pharmaceutical companies like Hoffmann-La Roche, known for breast and colon cancer treatments, and diagnostic companies such as Gen-Probe, known for its revolutionary diagnostic test for sextually transmitted diseases. ICBII was funded by Dr. Bhatt from its inception in October 2008 till December 2012 until ICBII had developed BBB permeable technology and proof-of-principle shown in animal model of Alzheimer’s disease by a third party, Dr. Eliezer Masliah, professor of Neuropathology, University of California, San Diego.
Q: Any highlights on your recent announcement?
A: ICBII: Our latest press release, dated September 22, 2021, highlighted the strengthening of ICBII’s patent portfolio with the approval of its 7th patent. The approval of its recent Blood-Brain Barrier Permeable Peptide Compositions covers the intellectual property for developing therapies that ICBII plans to market for reversing and potentially curing Alzheimer’s, Parkinson’s, and other neurological diseases.

Q: Can you give us more insights into your offering?
A: ICBII: We have been talking with multiple parties for partnering with us to conduct clinical trials and potentially manufacture and market drugs for Alzheimer’s, Parkinson’s, and Multiple System Atrophy. The purpose of the Press Release is to let our potential partners and investors know that ICBII is moving ahead rapidly to achieve its goal of revolutionizing the treatment of neurological diseases.
Q: What can we expect from your company in next 6 months? What are your plans?
A: ICBII: We expect to finalize an agreement with a major partner that will enable ICBII to proceed full steam ahead with plans for manufacturing our drugs following FDA outlined Good Manufacturing Procedure (GMP) and conducting multiple drug trials. In our labs, we have already developed SMART molecules for multiple disease targets, and we are looking for partners whose vision is aligned with ICBII.
Q: What is the best thing about your company that people might not know about?
A: ICBII: The best thing that people may not know about our Company is that our technology enables our antibodies/drugs to reach the brain with 150 times greater permeability than the classical mouse antibodies used by several big pharma companies for many decades. Again, brain diseases cannot be cured if the drug does not reach the target site in the central nervous system in therapeutically useful doses. The classical mouse monoclonal antibodies used in failed clinical trials by most pharma over the last 3-4 decades have blood-brain barrier permeability of about 0.1% in animals and humans. Such a low brain uptake cannot result in therapeutically effective doses, which naturally leads to failure in clinical trials. We have developed a novel class of antibodies with brain uptake of 15%, which is 150X higher than of classical mouse monoclonal antibodies used by big pharma. With superior brain uptake, our drugs are highly likely to modify and potentially cure central nervous system diseases. Our dream is to treat some of the most debilitating diseases in the world with a simple weekly injection, and we are moving closer to that dream every day.